Evolving Therapeutics in Multiple Myeloma
Although highly effective treatments are available in early-line settings of multiple myeloma management, such as proteasome inhibitors, immunomodulatory drugs (IMiDs), corticosteroids, and multiple drug combinations, including anti-CD38 antibodies, treatment options for progressive disease following initial lines of therapy have historically been limited.1,2
Available therapies for the management of relapsed/refractory multiple myeloma include many of the options used in earlier-line settings following treatment-free intervals. Others have been added, including exportin-1 (XPO1) inhibitors, signaling lymphocyte activation molecule family 7 (SLAMF7) antibodies, anti-CD38 antibodies, chimeric antigen receptor (CAR) T-cell therapies, and bispecific antibodies, which all may afford the opportunity to improve outcomes for patients with relapsed/refractory multiple myeloma.4 Several of these options, including CAR T-cell therapies and multiple bispecific antibodies, target B-cell maturation antigen (BCMA), which is expressed selectively on the surface of myeloma cells relative to normal plasma cells.5
Anti-CD38 antibodies
CD38 is a transmembrane glycoprotein that is expressed minimally on myeloid and lymphoid cells, but normal and malignant plasma cells have much higher levels of surface CD38 expression.6 Monoclonal antibodies directed against this protein are associated with multiple mechanisms of action that can attack the multiple myeloma cells, and two agents have been approved in the treatment of patients with multiple myeloma: daratumumab and isatuximab.6
In the POLLUX clinical trial, patients with relapsed or refractory multiple myeloma who received the combination of the anti-CD38 antibody daratumumab with lenalidomide and dexamethasone had significantly prolonged progression-free survival (PFS) relative to those who received lenalidomide and dexamethasone alone.7 Similarly, in the CASTOR clinical trial, patients with relapsed or refractory multiple myeloma who received the combination of daratumumab with bortezomib and dexamethasone had significantly prolonged PFS relative to those who received bortezomib and dexamethasone alone.8 Overall survival improvements in patients with relapsed/refractory multiple myeloma with daratumumab have also been observed in the CASTOR and POLLUX studies.9
Additionally, the anti-CD38 antibody isatuximab was evaluated in the ICARIA-MM study, in which patients with multiple myeloma who had received multiple lines of therapy were randomized to receive (1) pomalidomide/dexamethasone/isatuximab or (2) pomalidomide/dexamethasone. Patients in the isatuximab group had a greater overall response rate (ORR) and PFS after a median follow-up period of 12 months.10,11 In the IKEMA study, patients with multiple myeloma who had received one to three lines of previous therapy were randomized to the combination of isatuximab/carfilzomib/dexamethasone or carfilzomib/dexamethasone.12 Patients in the isatuximab group had a significantly greater PFS.12 Overall survival benefits have also been observed with the addition of isatuximab in a follow-up analysis of the ICARIA-MM study.13
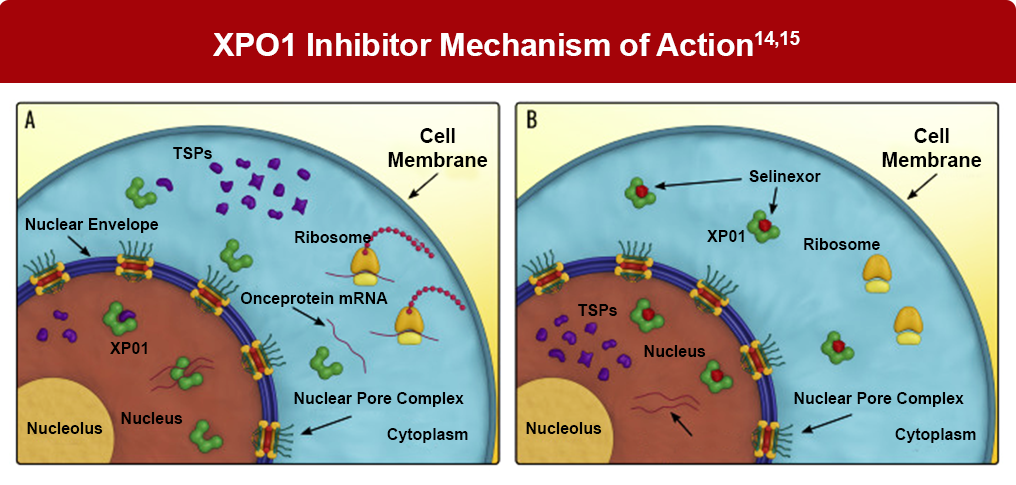
XPO-1 inhibitor therapy
The upregulation of exportin-1 (XPO1) inactivates tumor suppressor anti-neoplastic function, facilitating the transport of tumor suppressor proteins from the nucleus to the cytoplasm. As XPO1 is overexpressed in multiple myeloma, it has been identified as a treatment target in multiple myeloma. Selinexor inhibits XPO1 and has been approved for the treatment of patients with relapsed/refractory multiple myeloma.14
The exportin-1 inhibitor selinexor was evaluated in the phase 3 BOSTON study of selinexor, bortezomib, and dexamethasone therapy administered once weekly versus twice weekly doublet therapy with bortezomib and dexamethasone. Patients in this study had previously received one to three lines of therapy, including proteasome inhibitors.16 Median PFS was significantly extended (13.93 months vs 9.46 months; HR 0.70 [95% CI 0.53–0.93], P = .0075) in patients with multiple myeloma. Adverse events of grade 3 or 4 severity included thrombocytopenia, fatigue, anemia, and pneumonia.16
SLAMF7 antibody therapy
Signaling lymphocyte activation molecule family member 7, or SLAMF7, is highly expressed on malignant plasma cells in most patients with multiple myeloma.17 It is also expressed on a range of other cells, including normal plasma cells, natural killer (NK) cells, and CD8+ T cells. Its mechanism in multiple myeloma is believed to promote adhesion of multiple myeloma cells to bone marrow stromal cells, facilitating proliferation and survival of malignant cells.17
Elotuzumab is an anti-SLAMF7 antibody. In the phase 3 ELOQUENT-2 clinical trial in patients who had previously received one to three prior lines of therapy, PFS between groups favored elotuzumab at 1 year (68% vs 57%) and 2 years (41% vs 27%).19 Overall response rates were also significantly increased (79% vs 66%; P < .001). Common grade 3 or 4 adverse events included lymphocytopenia, neutropenia, fatigue, and pneumonia.19
Chimeric Antigen Receptor (CAR) T-cell therapy
CAR T-cell therapy is a method in which genetic manipulation of T-cells can introduce antigen-specific moieties that target myeloma cells.17
Two CAR-T cell therapies have been approved for the management of patients with relapsed/refractory multiple myeloma, including: ciltacabtagene autoleucel (cilta-cel) and idecabtagene vicleucel (ide-cel). Both cilta-cel and ide-cel were originally approved for later lines of therapy, after three and four prior therapies, respectively. In 2024, the FDA approved both CAR T-cell therapies for earlier use, after one prior therapy for cilta-cel and after two prior therapies for ide-cel.1,21,22,23
- Ciltacabtagene autoleucel: The CARTITUDE-4 study evaluated the safety and efficacy of the CAR T-cell therapy cilta-cel compared with standard care therapy in 419 patients with relapsed or refractory multiple myeloma who had received at least one previous line of therapy.23 The primary endpoint was PFS, which was not reached in the cilta-cel group and 11.8 months in the standard-care group (HR, 0.26; P < .001) after 15.9 months of follow-up.24 Other efficacy measures included ORR (84.6% vs 67.3%) and minimal residual disease negativity (60.6% vs 15.6%), and progression-free survival. Median PFS and OS were not reached at 27.7 months, with a PFS rate of 54.9% and an OS of (70.4%).24 Common adverse events associated with cilta-cel included any-grade cytokine release syndrome (CRS) (76.1%) and neurotoxicity (21%); however, grade 3 or 4 CRS (1.1%) and neurotoxicity (2.8%) were less common.23 Grade 3 or 4 blood dyscrasias were the most common serious adverse events with cilta-cel, and included neutropenia (89%), anemia (54.3%), thrombocytopenia (54.3%), and lymphopenia (22.1%).23 There were ten deaths in the cilta-cel group and 5 deaths in the standard care group that were due to treatment-related adverse events.24 Ciltacabtagene autoleucel is currently approved for adult patients who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to lenalidomide.25
- Idecabtagene vicleucel: In the phase 3 KarMMa-3 trial, 386 patients with triple-class exposed multiple myeloma who had received two to four previous therapies received idecabtagene vicleucel or one of five standard therapies.26 At 18.6 months of follow-up, the median PFS was 13.3 months for the ide-cel group versus 4.4 months for standard therapy (HR, 0.49; P < .001).26 Adverse events included CRS, which was observed at any grade in 88% of patients treated with ide-cel versus 0% of patients receiving standard therapy with 4% versus 0% of patients experiencing grade 3 or higher CRS. Neurotoxicity of any grade occurred in 15% versus 0% of patients, with 3% versus 0% of cases being of grade 3 or higher severity.26 Additional adverse events of grade 3 or higher included hematologic abnormalities, including neutropenia (76% vs 40%), anemia (51% vs 18%), and thrombocytopenia (42% vs 17%).26 The findings of this study led to the approval of ide-cel for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.27,28
Of note, both cilta-cel and ide-cel are available only through a Risk Evaluation and Mitigation Strategy restricted program.25,28
Bispecific antibody therapies
Bispecific T-cell antibodies have the capacity to bind both to a target moiety on myeloma cells and CD3 on T-cells simultaneously, facilitating direct T-cell activation and death of the tumor cells.29
Several bispecific antibodies have been approved for the management of relapsed/refractory multiple myeloma, including the anti-BCMA antibodies teclistamab, elranatamab, and linvoseltamab and the anti-GPRC5D (G protein-coupled receptor class 5 member D) antibody talquetamab. These therapies have similar indications, with all four agents approved for use in adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.31-33 Efficacy and safety data for each of these approved bispecific antibodies are reviewed below:
- Teclistamab: Teclistamab binds CD3 on T-cells and BCMA on myeloma cells. Its approval was granted based on results of the MajesTEC-1 study, in which 165 patients with relapsed/refractory multiple myeloma who received teclistamab were reported to have an ORR of 63% and a complete response rate or better of 39.4%.34 The median duration of response was 18.4 months, with a reported median PFS of 11.3 months.34 With respect to adverse events, 72.1% of patients experienced CRS, although most events were relatively mild (grade 1 or 2).34 One case of grade 3 CRS was reported, with no cases of grade 4 CRS.35 More than one-third of patients (36.4%) received supportive treatment of CRS with tocilizumab.34 The most common grade 3 or 4 events were neutropenia (64.2%), anemia (37.0%), lymphopenia (32.7%), and thrombocytopenia (21.2%).34 Other notable adverse events of any grade included neurotoxic events (14.5%) and infections (76.4%).34 With long-term follow-up at 30.4 months, the median duration of response was 24.0 months, with a median PFS of 11.4 months and median overall survival of 22.2 months.36 Grade 3 or 4 hematologic adverse events such as neutropenia (65%), anemia (38%), thrombocytopenia (23%), and lymphopenia (35%) were noted with long-term follow-up, along with infections (55%).36 No new safety signals were reported, and the incidence of new grade ≥3 infections decreased over time.36
For patients who have achieved and maintained complete response or better for at least 6 months, biweekly dosing of teclistamab may be considered.32 A biweekly dosing regimen of teclistamab was studied by Usmani et al, reporting high rates of complete response, with a median duration of response of 20.5 months.37 Real-world outcomes are similar to the results observed in trials, with one real-world study reporting an ORR of 48.9% and a very good partial response rate or better in 22.2% of patients ineligible for enrollment in MajesTEC-1.38 Additional real-world evidence has also favorably compared outcomes with teclistamab versus real-world physician’s choice of therapy in patients with triple-class exposed relapsed/refractory multiple myeloma.39 Teclistamab is being studied in combination with other treatment options: the combination of teclistamab with daratumumab and lenalidomide is being evaluated in the MajesTEC-2 multicohort study, reporting positive ORR findings and safety profile consistent with the individual components of the combination.40 Teclistamab is also being studied in combination with nirogacestat as part of the MajesTEC-2 study.41
- Talquetamab: Talquetamab is a bispecific antibody that binds to CD3 and GPRC5D to attack myeloma cells. In the phase 1/2 MonumenTAL-1 study, 297 patients with relapsed/refractory multiple myeloma (median 6 prior lines) received subcutaneous talquetamab. At recommended phase 2 doses, the overall response rate (ORR) was 74% (95% CI, 66–81) with 0.4 mg/kg weekly (n=143) and 73% (95% CI, 65–80) with 0.8 mg/kg every 2 weeks (n=154). Complete response or better occurred in 33% and 35%, respectively, and very good partial response or better in 59% and 60%. Median duration of response was 9.5 months (weekly) and 13.9 months (biweekly). CRS occurred in 79% (weekly) and 72% (biweekly) of patients, mostly grade 1–2. Non-hematologic adverse events included dysgeusia (57% weekly, 58% biweekly), skin-related events (56%, 51%), and nail disorders (49%, 44%). Grade 3–4 hematologic events were frequent: neutropenia (36%, 35%), anemia (31%, 28%), and lymphopenia (29%, 30%). No grade 5 CRS or ICANS was reported.42
In phase 2 results of the MonumenTAL-1 study analyzing patients who had received at least three prior lines of therapy, overall response rates were 74% (0.4 mg/kg weekly), 69% (0.8 mg/kg every 2 weeks), and 67% (prior T-cell redirection therapy).44 At a median follow up of 25.6 months, PFS was 5.1 months in patients who had received prior CAR T-cell therapy or bispecific antibody therapy.44 Talquetamab and teclistamab are being studied in combination with daratumumab in the TRIMM-2 study of patients with relapsed/refractory multiple myeloma.45,46
- Elranatamab: Elranatamab is a BCMA-directed bispecific antibody binding CD3 on T-cells, and it is approved for use in relapsed/refractory multiple myeloma.1,31 The phase 2 MagnetisMM-3 trial evaluated the use of weekly elranatamab in patients with relapsed/refractory multiple myeloma.47 The ORR in patients receiving elranatamab was 61%, with complete response or better observed in 35% of patients.47 For patients who switched to biweekly dosing, 80% improved or maintained their level of response for at least 6 months.47 Some of the common adverse events of any grade were infections (69.9%), CRS (57.7%), anemia (48.8%), and neutropenia (48.8%). No grade 3 or higher CRS events were reported.47
In a follow-up of the MagnetisMM-3 study, with a median follow-up of 28.4 months, the ORR was 61%, with a complete response or better rate of 37.4%.48 The median PFS was reported as 17.2 months, with a median OS of 24.6 months. No new safety signals were reported.48
- Linvoseltamab: Linvoseltamab is a BCMA×CD3 bispecific antibody that demonstrated efficacy and safety in the phase 1/2 LINKER-MM1 study of patients with relapsed/refractory multiple myeloma. The phase 2 portion of the study included patients who had received 3 or more previous lines of therapy, evaluating two full doses of linvoseltamab: 50 mg and 200 mg.49 For the 117 patients receiving 200 mg, including 39% with high-risk cytogenetics and 28% with pentarefractory disease, the ORR was 71%, and 50% achieved a complete response or better.49 The median duration of response was 29.4 months.49 For patients receiving 50 mg, the ORR was 48%, with 21% achieving a complete response or better. In patients receiving 200 mg, 46.2% of patients reported any degree of CRS; only 0.9% of patients at this dose experienced grade 3 CRS.49 Approximately 74% of patients receiving linvoseltamab reported infections.49 A phase 3 open-label study of linvoseltamab in patients with relapsed/refractory multiple myeloma has begun: the LINKER MM3 study is actively recruiting to compare outcomes between linvoseltamab and the combination of elotuzumab, pomalidomide, and dexamethasone.50 Linvoseltamab was granted accelerated FDA approval on July 2, 2025, based on the results of LINKER-MM1 and has been included in the NCCN Guidelines as a preferred regimen for relapsed/refractory multiple myeloma after progression on >4 lines of therapy, including an immunomodulatory drug, proteosome inhibitor, and anti-CD38 monoclonal antibody. Recommended dosing includes step-up doses of 5 mg, 25 mg, and 200 mg, followed by 200 mg weekly for 10 doses, then 200 mg biweekly. Patients who achieve and maintain a VGPR or better by week 24 can transition to monthly dosing.51
- ABBV-383 (etentamig): ABBV-383 is a BCMA-directed bispecific antibody that has reported safety and efficacy in an early-phase study. A phase 1 trial of patients with relapsed/refractory multiple myeloma who received three or more lines of therapy reported that patients receiving ABBV-383 had an objective response rate of 57%, with a very good partial response or better rate of 43%.52 Approximately 57% of patients experienced CRS of any degree.52 With respect to hematologic treatment-emergent adverse events, approximately 29% of patients experienced anemia and 37% of patients experienced neutropenia with ABBV-383.51 Phase 3 investigation with ABBV-383 has begun.53
- Antibody-Drug Conjugates (ADCs): Belantamab mafodotin is an ADC comprising a BCMA-targeted monocolonal antibody conjugated to a monomethyl auristatin F payload via a noncleavable linker. This configuration enhances antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis, thereby strengthening the immune response against BCMA-expressing multiple myeloma cells. In the phase 3 DREAMM-7 trial, belantamab mafodotin plus bortezomib and dexamethasone (BVd) was compared with daratumumab plus bortezomib and dexamethasone (DVd) in 494 patients with RRMM that had progressed on > 1 prior lines of therapy (median follow-up, 28.2 months). Median PFS was significantly improved with BVd versus DVd (36.6 months vs 13.4 months; HR, 0.41; P < .001). However, grade > 3 AEs were reported in 95% of the patients in the BVd group, and serious adverse events occurred in 50% of BVd-treated patients, with 25% discontinuing therapy due to toxicity. Prescribing information for BVd includes a Boxed Warning for ocular toxicity, including corneal vision deterioration. In DREAMM-7, ocular toxicity occurred in 92% of patients, including grade 3/4 in 77%.56,57,58
The increasing range of mechanisms available in the relapsed/refractory setting of multiple myeloma has greatly broadened the available regimens for the later-line management of disease; however, the large number of classes of therapies available has also introduced uncertainties in how to select and apply therapy. Major advances in the management of relapsed/refractory multiple myeloma include BCMA-targeting treatments such as the CAR-T therapies ciltacabtagene autoleucel and idecabtagene vicleucel, and the bispecific antibodies teclistamab, elranatamab, and linvoseltamab. Careful consideration of the safety and efficacy profiles of these agents will help to optimize outcomes in patients who may be eligible for these therapies.
References
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3.2026. Published November 3, 2025. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- Dimopoulos MA, Richardson P, Lonial S. Treatment options for patients with heavily pretreated relapsed and refractory multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2022;22:460-473.
- Su CT, Ye JC. Emerging therapies for relapsed/refractory multiple myeloma: CAR-T and beyond. J Hematol Oncol. 2021;14:115.
- American Cancer Society. Drug Therapy for Multiple Myeloma Last revised: October 27, 2025. https://www.cancer.org/cancer/types/multiple-myeloma/treating/chemotherapy.html
- Sammartano V, Franceschini M, Fredducci S, et al. Anti-BCMA novel therapies for multiple myeloma. Cancer Drug Resist. 2023;6:169-181.
- De Novellis D, Fontana R, Guidice V, et al. Innovative anti-cd38 and anti-bcma targeted therapies in multiple myeloma: Mechanisms of action and resistance. Int J Mol Sci. 2023;24:645.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
- Dimopoulos MA, Oriol A, Nahi H, et al. Overall survival with daratumumab, lenalidomide, and dexamethasone in previously treated multiple myeloma (POLLUX): A randomized, open-label, phase III Trial. J Clin Oncol. 2023;41:1590-1599.
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096-2107.
- Bringhen S, Pour L, Vorobyev V, et al. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma according to prior lines of treatment and refractory status: ICARIA-MM subgroup analysis. Leuk Res. 2021;104:106576.
- Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. 2021;397:2361-2371.
- Richardson PG, Perrot A, San Miguel JS, et al. Isatuximab-pomalidomide-dexamethasone versus pomalidomide-dexamethasone in patients with relapsed and refractory multiple myeloma: Final overall survival analysis. Haematologica. 2024;109:2239-2249.
- Mo CC, Yee AJ, Midha S, et al. Selinexor: Targeting a novel pathway in multiple myeloma. EJHaem. 2023;4:792-810.
- Benkova K, Mihalyova J, Hajek R, Jelinek T. Selinexor, selective inhibitor of nuclear export: Unselective bullet for blood cancers. Blood Rev. 2021;46100578.
- Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. 2020;396:1563-1573.
- Al-Hujaily EM, Oldham RAA, Hari P, Medin JA. Development of novel immunotherapies for multiple myeloma. Int J Mol Sci. 2016;17:1506.
- Chu E, Wu J, Kang SS, Kang Y. SLAMF7 as a promising immunotherapeutic target in multiple myeloma treatments. Curr Oncol. 2023;30:7891-7903.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
- UT Southwestern Medical Center. New CAR T-cell therapy extends remission in heavily relapsed multiple myeloma. March 8, 2021. https://www.utsouthwestern.edu/newsroom/articles/year-2021/new-car-t-cell-therapy.html
- US Food & Drug Administration (FDA). FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. February 28, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma
- National Institutes of Health (NIH). National Cancer Institute. FDA approves BCMA-targeted CAR T-cell therapy for multiple myeloma. April 14, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2021/fda-ide-cel-car-t-multiple-myeloma
- After ODAC Review: FDA Approves Abecma and Carvykti in Earlier Lines of Therapy for Relapsed or Refractory Myeloma Patients. International Myeloma Foundation. April 11, 2024. https://www.myeloma.org/blog/dr-duries/fda-approves-abecma-and-carvykti-in-earlier-treatment-of-RRMM
- San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023;389:335-347.
- Carvykti® (ciltacabtagene autoleucel). Prescribing information. Janssen Biotech Inc; 2025. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/CARVYKTI-pi.pdf
- Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2023;388:1002-1014.
- US Food & Drug Administration (FDA). FDA approves idecabtagene vicleucel for multiple myeloma. March 26, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma
- Abecma® (idecabtagene vicleucel). Prescribing information. Bristol Myers Squibb; 2025. https://packageinserts.bms.com/pi/pi_abecma.pdf
- Swan D, Murphy P, Glavey S, Quinn J. Bispecific antibodies in multiple myeloma: Opportunities to enhance efficacy and improve safety. Cancers (Basel). 2023;15:1819.
- The Patient Story. The Future of Multiple Myeloma Treatment: Expert Q&A on Bispecific Antibodies. https://thepatientstory.com/medical-experts/the-role-of-bispecific-antibodies-in-the-treatment-of-multiple-myeloma/
- ElrexfioTM (elranatamab-bcmm). Prescribing Information. Pfizer Inc; 2025. https://labeling.pfizer.com/ShowLabeling.aspx?id=19669
- Tecvayli® (teclistamab-cqyv). Prescribing Information. Janssen Biotech, Inc; 2025. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TECVAYLI-pi.pdf
- Talvey® (talquetamab-tgvs). Prescribing Information. Janssen Biotech, Inc; 2025. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TALVEY-pi.pdf
- Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495-505.
- Martin TG, Mateos MV, Nooka A, et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer. 2023;129:2035-2046.
- Garfall AL, Nooka AK, van de Donk NWCJ, et al. Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42(16_suppl):7540.
- Usmani SZ, Karlin L, Benboubker L, et al. Durability of responses with biweekly dosing of teclistamab in patients with relapsed/refractory multiple myeloma achieving a clinical response in the majesTEC-1 study. J Clin Oncol. 2023;41(16_suppl):8034.
- Gordon B, Fogel L, Varma G, et al. Teclistamab demonstrates clinical activity in real-world patients ineligible for the Pivotal Majestec-1 trial. Blood. 2023;142(suppl 1):4741.
- Krishnan A, Nooka AK, Chari A, et al. Teclistamab versus real-world physician’s choice of therapy in triple-class exposed relapsed/refractory multiple myeloma. J Comp Eff Res. 2023;12:e220186.
- Searle E, Quach H, Wong SW, et al. Teclistamab in combination with subcutaneous daratumumab and lenalidomide in patients with multiple myeloma: Results from one cohort of MajesTEC-2, a phase 1b, multicohort study. Blood. 2022;140(suppl 1):394-396.
- Offner F, Decaux O, Hulin C, et al. S194 Teclistamab (TEC) + nirogacestat (NIRO) in relapsed/refractory multiple myeloma (RRMM): The phase 1B MAJESTIC-2 study. Hemasphere. 2023;7(suppl 3):e1257964.
- Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387:2232-2244.
- Liu L, Krishnan A. Talquetamab in multiple myeloma. Haematologica. 2024;109:718-724. org/10.3324/haematol.2023.283931
- Chari A, Touzeau C, Schinke C, et al. Safety and activity of talquetamab in patients with relapsed or refractory multiple myeloma (MonumenTAL-1): A multicentre, open-label, phase 1-2 study. Lancet Haematol. 2025;12:e269-e281.
- Rodriguez-Otero P, D’Souza A, Reece DE, et al. A novel, immunotherapy-based approach for the treatment of relapsed/refractory multiple myeloma (RRMM): Updated phase 1b results for daratumumab in combination with teclistamab (a BCMA x CD3 bispecific antibody). J Clin Oncol. 2022;40(16_suppl):8032.
- Dholaria BR, Weisel K, Mateos MV, et al. Talquetamab (tal) + daratumumab (dara) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Updated TRIMM-2 results. J Clin Oncol. 2023;41(16_suppl):8003.
- Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29:2259-2267.
- Tomasson MH, Iida S, Niesvizky R, et al. Long‐term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM‐3 study. Hemasphere. 2024;8:e136.
- Bumma N, Richter J, Jagannath S, et al. Linvoseltamab for treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42:2702-2712.
- A Trial to Learn How Well Linvoseltamab Works Compared to the Combination of Elotuzumab, Pomalidomide and Dexamethasone for Adult Participants With Relapsed/Refractory Multiple Myeloma (LINKER-MM3). ClinicalTrials.gov identifier: NCT05730036. Last updated: October 30, 2025. https://clinicaltrials.gov/study/NCT05730036
- FDA grants accelerated approval to linvoseltamab-gcpt for relapsed or refractory multiple myeloma. June 2, 2025.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-linvoseltamab-gcpt-relapsed-or-refractory-multiple-myeloma
- D’Souza A, Shah N, Rodriguez C, et al. A phase I first-in-human study of ABBV-383, a B-cell maturation antigen x CD3 bispecific T-cell redirecting antibody in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2022;40:3576-3586.
- A Phase 3, Multicenter, Randomized, Open Label Study of ABBV-383 Compared With Standard Available Therapies in Subjects With Relapsed or Refractory Multiple Myeloma (3L+ RRMM Monotherapy Study). ClinicalTrials.gov. identifier: NCT06158841. Last updated October 28, 2025. https://clinicaltrials.gov/study/NCT06158841
- Sun M, Qui L, Wei Y, et al. Results from a first-in-human phase I study of F182112, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023;41(16_suppl):8038.
- Suvannasankha A, Kapoor P, Pianko MJ, et al. Abstract CT013: Safety and efficacy from the phase 1/2 first-in-human study of REGN5459, a BCMA×CD3 bispecific antibody with low CD3 affinity, in patients with relapsed/refractory multiple myeloma. Cancer Res. 2023;83(8_suppl):CT013.
- Almodovar Diaz AA, Alouch SS, Chawla Y, Gonsalves WI. The antibody drug conjugate, belantamab-mafodotin, in the treatment of multiple myeloma: A comprehensive review. Blood Lymphat Cancer. 2024;14:71-87.
- FDA approves belantamab mafodotin-blmf for relapsed or refractory multiple myeloma. October 23, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belantamab-mafodotin-blmf-relapsed-or-refractory-multiple-myeloma
- Hungria V, Robak P, Hus M, et al. Belantamab mafodotin, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2024;391:393-407.
All URLs accessed November 4, 2025