What are Antibody Therapies?
Antibody therapies augment the naturally occurring antibodies in our immune system that help the immune system recognize infections.1-3 Antibodies have been developed to help identify and attack malignant cells in multiple myeloma and in other cancers. Multiple novel combinations of antibody therapeutics are now available for the management of multiple myeloma. These include:
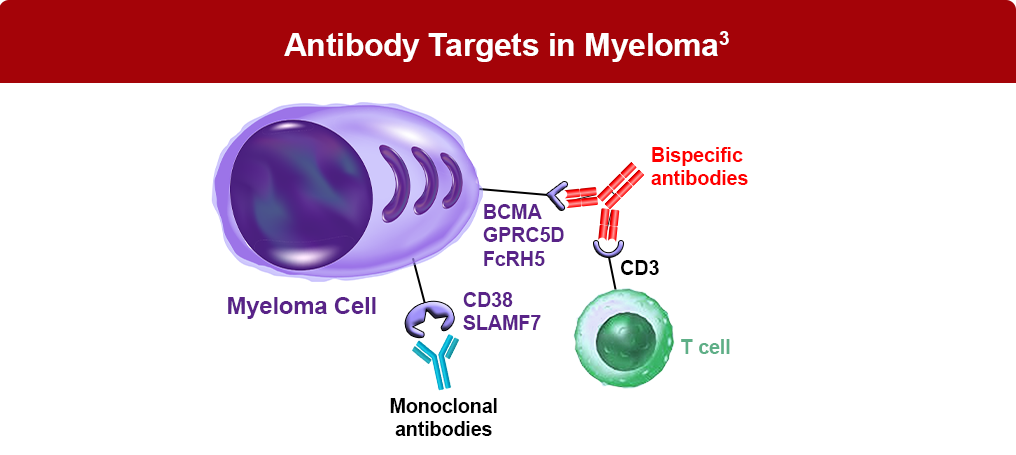
- Anti-CD38 antibodies
- Signaling lymphocyte activation molecule family 7 (SLAMF7) antibodies
- B-cell maturation antigen (BCMA)-targeted antibody-drug conjugates
- Bispecific antibodies targeting BCMA x CD3 and GPRC5D x CD3
Bispecific antibodies can help bring together T cells, which are immune cells involved in targeting and destroying cancer cells, and specific receptors on cancer cells. In this way, bispecific antibodies can help the immune system recognize and destroy cancer.
One key target on cancer cells in multiple myeloma is BCMA, which is generally expressed to a greater degree on abnormal plasma cells than on normal plasma cells.4 Three bispecific antibodies targeting BCMA have been approved for the treatment of multiple myeloma, including teclistamab, elranatamab, and linvoseltamab. Another bispecific antibody, talquetamab, targets GPRC5D.1 Other bispecific antibodies targeting BCMA are in active development for multiple myeloma5-10, including:
- ABBV-383 (etentamig) (phase 3)
- REGN 5459 (phase 1/2)
- EMB-06 (phase 1/2)
References
- American Cancer Society. Drug Therapy for Multiple Myeloma. Last Revised: October 27, 2025 https://www.cancer.org/cancer/types/multiple-myeloma/treating/chemotherapy.html
- D’Agostino M, Innorcia S, Boccadoro M, Bringhen S. monoclonal antibodies to treat multiple myeloma: A dream come true. Int J Mol Sci. 2020;21:8192.
- Lin CHT, Tarig MJ, Ullah F, et al. Current novel targeted therapeutic strategies in multiple myeloma. Int J Mol Sci. 2024;25:6192.
- Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: Potential uses of BCMA-based immunotherapy. Front Immunol. 2018;9:1821.
- A Trial to Learn How Well Linvoseltamab Works Compared to the Combination of Elotuzumab, Pomalidomide and Dexamethasone for Adult Participants With Relapsed/Refractory Multiple Myeloma (LINKER-MM3). ClinicalTrials.gov identifier: NCT05730036. Last updated October 30, 2025. https://clinicaltrials.gov/study/NCT05730036
- Bumma N, Richter J, Jagannath S, et al. Linvoseltamab for treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42:2702-2712.
- First In Human (FIH) Study of REGN5459 in Adult Patients With Relapsed or Refractory Multiple Myeloma (MM). ClinicalTrials.gov identifier: NCT04083534. Last updated: May 30, 2025. https://clinicaltrials.gov/study/NCT04083534
- Suvannasankha A, Kapoor P, Pianko MJ, et al. Safety and efficacy from the phase 1/2 first-in-human study of REGN5459, a BCMAxCD3 bispecific antibody with low CD3 affinity, in patients with relapsed/refractory multiple myeloma. Cancer Res. 2023;83(8_suppl):CT013.
- Study Assessing Activity of Intravenous (IV) Etentamig Monotherapy Versus Standard Available Therapies in Adult Participants With Relapsed or Refractory Multiple Myeloma (CERVINO). ClinicalTrials.gov identifier: NCT06158841. Last updated: October 28, 2025. https://clinicaltrials.gov/study/NCT06158841
- A Ph1/2 Study of EMB-06 in Participants With Relapsed or Refractory Myeloma. ClinicalTrials.gov identifier: NCT04735575. Last updated May 28, 2025. https://clinicaltrials.gov/study/NCT04735575
- National Comprehensive Cancer Network®. NCCN Guidelines for Patients. Multiple Myeloma, 2025. https://www.nccn.org/patients/guidelines/content/PDF/myeloma-patient.pdf
ALL URLs accessed October 27, 2025